SARS-CoV-2
Reliable detection of SARS-CoV-2 with two target genes
Why choose our test?
The GenomEra® SARS-CoV-2 Assay Kit is a rapid and affordable multiplex reverse transcriptase polymerase chain reaction (RT-PCR) test for the detection of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) from respiratory specimens. RNA extraction is not needed, dramatically reducing the hands-on time and workload of the laboratory personnel and allowing for timely results. The easy-to-use GenomEra CDX System combines the simplicity of rapid testing and the superior sensitivity of PCR methods in one reliable system.
SARS-CoV-2 belongs to the family of Coronaviridae within the Betacoronaviruses genus and is an enveloped, positive-sense single-stranded RNA (+ssRNA) virus of zoonotic origin.1 SARS-CoV-2 is closely related to the members of a viral species termed severe acute respiratory syndrome-related CoV (SARSr-CoV), a species defined as the agent of the 2002/03 outbreak of SARS in humans.2,3 SARS-CoV-2 is the cause of the COVID-19 pandemic that was designated a public health emergency by the World Health Organization (WHO) in March 2020.4,5
Human-to-human transmission occurs primarily via respiratory droplets from coughs and sneezes.6 Indirect contact via contaminated surfaces is another possible cause of infection7, and viral RNA has also been found in the stool of infected people.8 Most infected people develop mild to moderate symptoms of fever, cough, tiredness, and shortness of breath, and recover without requiring special treatment. Individuals with underlying medical conditions and those over 60 years of age have a higher risk of severe, life-threatening disease.9
Although home tests are needed to accelerate clinical decision-making and to alleviate the workload of centralized test laboratories, RT-PCR is the standard approach for COVID-19 screening in symptomatic patients and also the on recommended by the WHO.10
Key facts
- Accurate RT-PCR detection of viral SARS-CoV-2 RdRp and E genes
- Results from four specimens in 70 minutes with no need for RNA extraction
- Highly cost-effective and adaptable for different types of health care facilities
Assay principle
The GenomEra SARS-CoV-2 Assay Kit is a rapid qualitative method for the molecular diagnostics of SARS-CoV-2 in respiratory specimens. The GenomEra® SARS-CoV-2 Assay Kit utilizes real-time RT-PCR and hydrolysis probes to detect the unique sequence regions of ARS-CoV-2 envelope (E) and RNA-dependent RNA polymerase (RdRP) protein-coding genes.
Respiratory swab specimens are collected and stored in Copan eSwab™, Universal Transport Medium (UTM), saline or VACUETTE® Virus Stabilization Tubes (PBS, phosphate buffered saline). Sample preparation is simple and requires only a quick heating step. The GenomEra Test Chips contain all the reagents needed for RT-PCR, which enables testing in laboratories without any prior experience in PCR diagnostics. The GenomEra SARS-CoV-2 assay includes a sample processing and amplification control, a small amount of MS2 bacteriophage that mimics the analyte viruses and contains a specific RNA sequence detected during the assay run.
The GenomEra SARS-CoV-2 Assay Kit is intended to aid the diagnosis of SARS-CoV-2 infection in humans when used in conjunction with clinical evaluation, patient history, and epidemiological information.
Test procedure
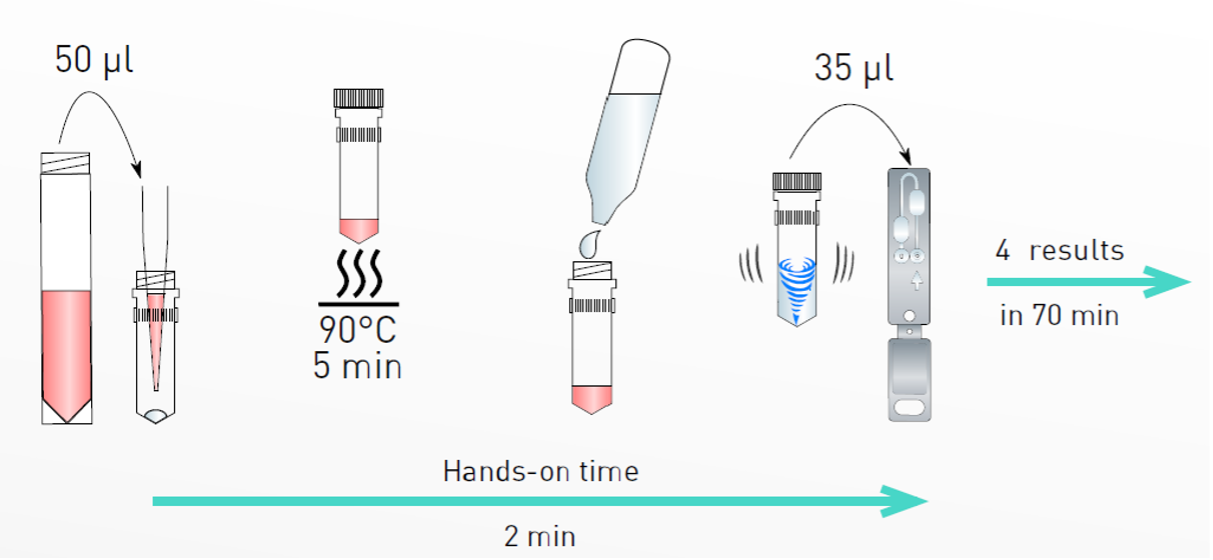
Clinical performance
The clinical performance of the GenomEra SARS-CoV-2 Assay Kit was evaluated at four institutions across Europe during the COVID-19 pandemic in June-July 2020 (Table 1). Fresh (prospective) and frozen (retrospective) specimens were collected in eSwab, UTM or 0.9% NaCl from patients with signs and symptoms of a respiratory infection. The reference methods used were standard RT-PCR methods developed for SARS-CoV-2 detection.
Table 1. The GenomEra SARS-CoV-2 Assay Kit is highly sensitive and specific in prospective and retrospective clinical specimens. NPA, negative percent agreement; PPA, positive percent agreement.
| Specimen type | Sample size, n | PPA, % (CI 95%) |
NPA, % (CI 95%) |
| Fresh | 180 | 80.4 (66.1–90.6) |
100 (97.3–100) |
| Frozen | 90 | 98.4 (91.3–100) |
100 (87.7–100) |
| Total | 270 | 90.7 (83.6–95.5) |
100 (97.8–100) |
Ordering information
To place an order, please contact your local distributor.
| Product name | Product code |
| GenomEra SARS-CoV-2 Assay Kit | |
| 20 tests | CDX-130-01-20 |
| 40 tests | CDX-130-01-40 |
| 100 tests | CDX-130-01-100 |
| GenomEra CDX System | CDX-10-020 |
The GenomEra SARS-CoV-2 Assay Kit has received the European CE marking for In Vitro Diagnostic (IVD) medical devices according to the requirements of EU Directive 98/79/EC (IVDD) and is available in European markets. In other markets, please contact your local distributor for availability. The product is manufactured by Uniogen, Finland.
References
- Zhang Y-Z. Novel 2019 coronavirus genome. [Accessed 25 Mar 2020].
- de Groot RJ, Baker SC, Baric R, Enjuanes L, Gorbalenya AE, Holmes KV, et al. Family Coronaviridae. In: King AMQ, Adams MJ, Carstens EB, Lefkowitz EJ. Virus taxonomy: classification and nomenclature of viruses: ninth report of the International Committee on Taxonomy of Viruses. London; Waltham: Academic Press; 2012. p. 806-820.
- Peiris JS, Yuen KY, Osterhaus AD, Stöhr K. The severe acute respiratory syndrome. N Engl J Med. 2003;349(25):2431-2441.
- Wee SL, McNeil Jr DG, Hernández JC (30 January 2020). “W.H.O. Declares Global Emergency as Wuhan Coronavirus Spreads”. The New York Times. Retrieved 25 Mar 2020.
- Chan JF, Yuan S, Kok KH, To KK, Chu H, Yang J et al. (February 2020). “A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster”. The Lancet. 395 (10223): 514–523.
- “How COVID-19 Spreads”. U.S. Centers for Disease Control and Prevention (CDC). 4 March 2020. Retrieved 25 March 2020.
- “Getting your workplace ready for COVID-19″ (PDF). World Health Organization (WHO). 3 March 2020. Retrieved 25 March 2020.
- Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H et al. (March 2020). “First Case of 2019 Novel Coronavirus in the United States”. The New England Journal of Medicine. 382(10): 929–936.
- “Coronavirus disease (COVID-19)”. World Health Organization (WHO). 2022. Retrieved 5 January 2022.
- Recommendations for national SARS-CoV-2 testing strategies and diagnostic capacities. World Health Organization (WHO). Interim guidance 25 June 2021. WHO REFERENCE NUMBER: WHO-2019-nCoV-lab-testing-2021.1-eng. Retrieved 5 January 2022.